Sodium formaldehydesulfoxylate,1 first reported in the chemical literature in 1905 and marketed as Rongalite (Figure 1, CAS: 149-44-0, dihydrate: 6035-47-8, from the French word rongeage for decolorize)- is a commodity chemical used in the textile and dye industry as a bleaching agent and as a reducing agent in redox initiator systems for polymer formation. Historically it has also been used as an antidote for heavy metal poisoning (it’s a good metal chelator) and as a photographic developing agent. The compound is manufactured by reaction of sodium dithionite with formaldehyde and is a crystalline, very hygroscopic solid that has a “faint leek” smell. A review by Kotha et al alerts the reader that “The loss of purity and hence reactivity is indicated if it smells like fish”.1 I wouldn’t advocate sniffing anything in the lab, particularly something that generates toxic gases on contact with acid and causes eye and skin irritation. Best engage you analytical colleagues if your concerned about purity ! It has many applications in synthetic chemistry,2 including methodology described in a recent publication that caught my attention on aryl radical generation and metal-free cross coupling reactions (more on this below).3 Here I give a brief overview of some of its useful applications and reasons why you might want to add it to your synthetic “Reagent of the month” toolbox.4
The most common applications of the reagent in synthetic chemistry are as a source of sulfoxylate (SO2– ) anion for the synthesis of sulfones, sultines, sulphonamides and sulfonyl fluorides. Other applications include promotion of SET reactions and as a reducing agent, both stand alone and in combination with tellurium (generating Na2Tex in situ). There are a few papers describing rongalite as source of SO2 (see the sulfonylhydrazide (Scheme 8) example below) and also in situ formation of HCHO and its use as a C-1 unit in the synthesis of heterocycles. A few examples of these useful transformations are shown below.
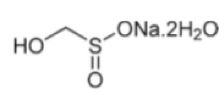
Alkylation of primary and secondary alkyl halides with rongalite has been demonstrated by a team at Pfizer, Groton as a one-pot telescoped process (Scheme 1).5 Acid or base induced fragmentation of a initially alkylated intermediate generates formaldehyde and an alkyl sulfinate that can be trapped to give the sulfone, sulphonamide or sulfonyl fluoride, all of which are useful building blocks. Both the sulphonamide and sulfone motifs are present in a number of pharmaceutical and agrochemical compounds including Tirofiban and Vermurafenib.6 Reaction of rongalite with activated vinyl derivatives such as acrylates gives the corresponding sulfone derivative in good yield via Michael-type addition chemistry.7
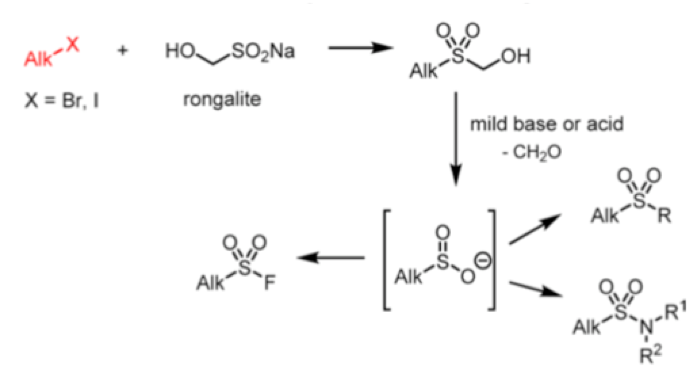
Reaction of rongalite with dihalides can be used to prepare sultines, or cyclic sulfinc esters. Sultines behave chemically in a similar way to their carbocyclic analoguesand undergo nucleophilic substitution at sulfur.They are used as lactone bioisosteres and also found in Nature. They are used extensively in the perfume industry.8
As synthetic precursors sultines can undergo thermal Diels-Alder reactions via generation of intermediate dienes (Scheme 2). Subsequent trapping with suitable dienophiles generates [4+2] cycloadducts or, in some cases, reaction with the extruded SO2 yields the sulfone.9
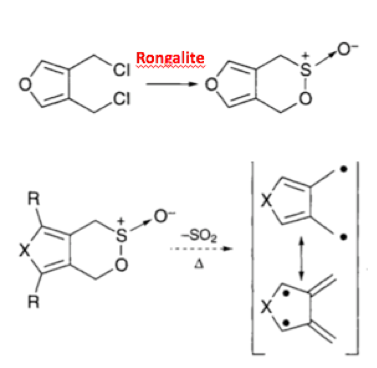
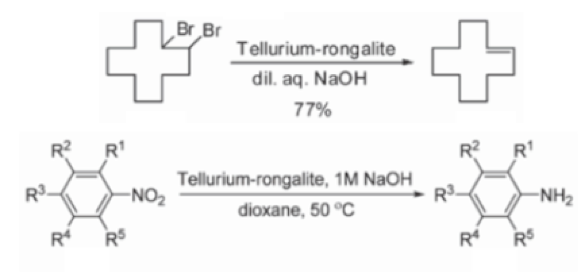
Reduction chemistry facilitated by rongalite is also well known. The combination of tellurium metal with the reagent in dilute aqueous sodium hydroxide generates a wine-red coloured solution containing Na2Te (or more precisely Na2TeX). Synthetic applications of this mixture include debromination of vicinal dibromoalkanes (used as an interesting protecting group strategy to mask and subsequently de-mask a double bond)10 and aryl nitro group reduction in which catalytic tellurium, in combination with excess alkaline rongalite, gives the corresponding aniline in good yield, allegedly without formation of azo, azoxy and hydrazo impurities- a common impurity in aryl nitro reduction reactions (Scheme 3).11
Rongalite is also a stand-alone reagent for reductive dehalogenation of a-halo ketones and aldehydes and perfluoro aryl halides. In addition reduction of aromatic aldehydes and benzoins to the corresponding alcohols in DMF as solvent at elevated temperatures has also been reported. Mechanistically it is believed the initial step is nucleophilic attack of SO22- or HOCH2SO2–on the carbonyl group.12
Thermal decomposition of rongalite generates formaldehyde, a C-1 fragment that has been employed in the synthesis of tri-substituted furans from aryl ketones using Cu and I2in DMSO solvent (Scheme 4).13
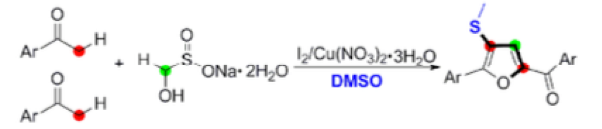
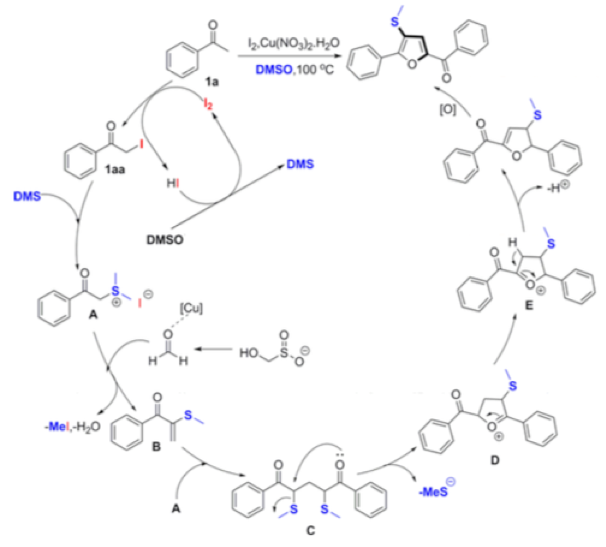
The suggested mechanism is somewhat complex, involving iodination of the ketone, reaction with DMS (generated from reduction of DMSO facilitated by HI) to form the sulfonium salt and subsequent trapping of the sulfur species with formaldehyde (formed by copper assisted decomposition of rongalite) to give a vinyl thioether! Subsequent dimerization and intramolecular cyclization gives, upon oxidation, the tri-substituted furan (Scheme 4).
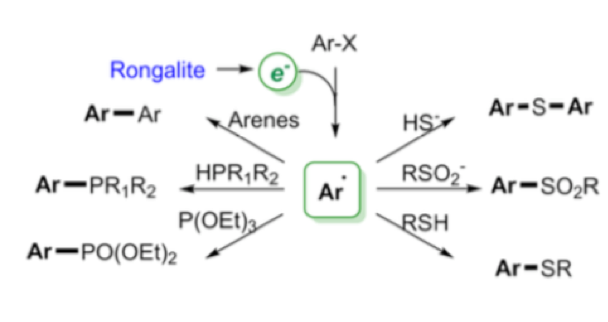
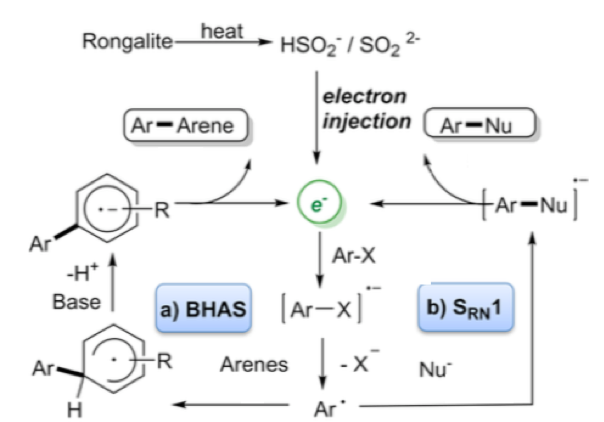
Perhaps the most interesting application of this reagent is described in a recent paper by Wang et al who essentially describes the use of the sulfur-containing degradation product (HOCH2SO2Na —-> CH2O + HSO2–/SO22-) as a super electron donor to generate aryl radicals and convert them into a range of arylated products without the use of transition metals (Scheme 5).3 These so called “electron catalysed reactions” can be utilised under basic conditions to generate biarylated products (BHAS) or trapped with a suitable nucleophile generating C-S or C-P bonds via a SRN1 electron catalysed process (Scheme 6). ESR experiments confirmed the presence of radicals under the reaction conditions and addition of TEMPO radical shut down the process. Additional trapping experiments also confirmed the radical pathway.
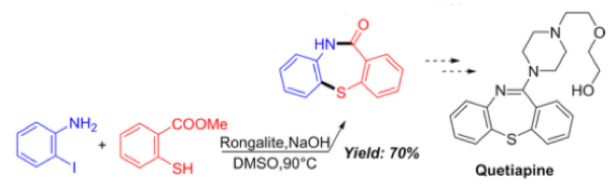
It’s always good to see application of new methodology directed toward a real-world target. In this case a 1-step synthesis of a key intermediate for the antipsychotic drug Quetiapine is exemplified in 70% yield (Scheme 7).
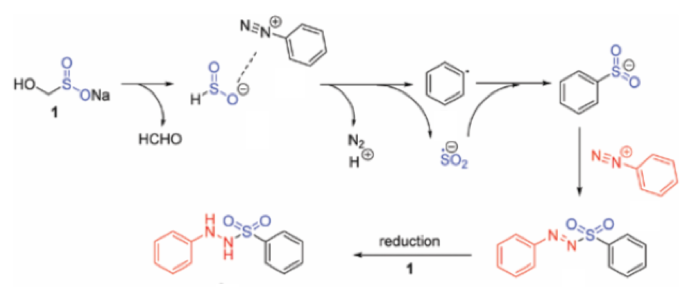
Another application of the HSO2–anion, generated by decomposition of rongalite, is the reaction with an aryl diazonium cation (Scheme 8). Formation of an aryl radical and a sulfoxylate radical anion by SET, radical coupling and subsequent reaction with another molecule of diazonium intermediate gives the corresponding sulfonyl hydrazide.14 This is a rare example of rongalite being used as a source of SO2 in synthesis.
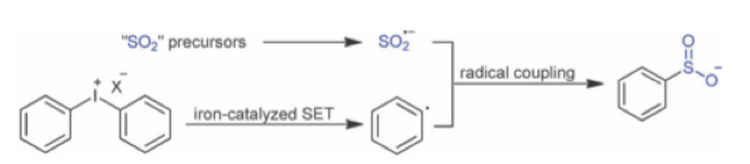
Another paper published by Luo et al, in which sulfoxylate radical anions are generated from rongalite and coupled with diaryliodonium under iron catalysis reports formation of aryl sulfinates and a limited set of sulphonamides (Scheme 9).15
I hope this has convinced you that this simple reagent has much potential in synthetic chemistry. It’s a cheap commodity chemical and well worth keeping on the shelf for those Friday afternoon experiments.
See you next time.
Written by John Studley, 1 Sept 2019
References:
- Rongalite: A useful green reagent in organic synthesis: S. Kotha et al, Chem. Rev. 2012, 112, 1650-1680. A zinc salt is also known (CAS: 24887-06-7).
- Synthetic applications of rongalite: A green tool in the service of Diels–Alder chemistry and beyond: S. Kotha et al, Tett. Lett. 2019, 60, 631-648.
- Generation of aryl radicals from aryl halides: rongalite-promoted transition-metal-free arylation: Y. Wang et al, J. Org. Chem. 2019, 84, 9946-9956.
- Follow our blog at www.scientificupdate.com/blog for past and future “reagent of the month” posts.
- Reaction of alkyl halides with rongalite: one-pot and telescoped syntheses of aliphatic sulfonamides, sulfonyl fluorides, and unsymmetrical sulfones: A. Shavnya et al, Org. Lett. 2016, 18, 5848-5851.
- Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery- A critical review: H. Qin et al, Eur. J. Med. Chem. 2019, 162, 679-734.
- R. Kerber et al, Chem. Ber. 1971, 104, 2035-2043.
- Sultones and sultines via a Julia–Kocienski reaction of epoxides: C. Bray et al, Angew. Chem. Int. Ed. 2015, 54, 15236-15240 (references within).
- D. Dittmer et al, J. Org. Chem.1991, 56, 1948.
- A mild and efficient debromination of vicinal dibromoalkanes with sodium telluride prepared from tellurium and rongalite: I. Masahiko et al, Chem. Lett. 1985, 14, 225-228.
- Reduction of aromatic nitro compounds with sodium telluride: I. Masahiko et al, Chem. Lett. 1985, 14, 1671-1674; Recent trends in the chemistry of sulfur-containing reducing agents: S. Makarov Russ. Chem. Rev. 2001, 70, 885-895.
- Sodium formaldehyde sulphoxylate an efficient reagent for the reduction of α-haloketones: A. Harris, Synth. Comm. 1987, 17, 1587-1592; Dittmer et al, J. Org. Chem. 1988, 53, 5750; S. Tsuboi et al, Tetrahedron 2007, 63, 970; The reduction of aromatic aldehydes and benzils by sodium formaldehyde sulphoxylate: A. Harris et al, Synth. Comm. 1989, 19, 529-535; Addition reactions of dibromodifluoromethane promoted by sulfinatodehalogenation reagents: F.Wu et al, J. Fluorine Chem. 1996, 80, 91-94.
- Synthesis of 2,4,5-trisubstituted furans via a triple C(sp3)-H functionalization reaction using rongalite as the C1 unit: A. Wu et al, Org. Lett. 2016, 18, 524-527.
- The triple role of rongalite in aminosulfonylation of aryldiazonium tetrafluoroborates: synthesis of N-aminosulfonamides via a radical coupling reaction: A. Wu et al, Chem. Commun. 2018, 54, 7641-7644.
- Iron-catalyzed synthesis of arylsulfinates through radical coupling reaction: M. Luo et al, Chem. Commun. 2016, 52, 2980-2983.








