In this post I’m highlighting a couple of papers that are essentially two sides of the same coin. As a process chemist, I’ll be the first to admit that the chemistry I’m about to describe is not going to end up in your manufacturing route any time soon, but as a former card-carrying medicinal chemist they would have been very useful to me at that time.
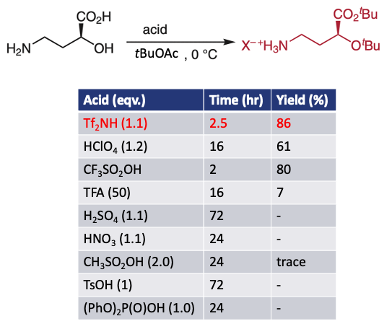
The first paper by Namba and his team at Tokushima University in Japan describes a simple method for direct conversion of free amino acids to their corresponding tert-butyl esters (Figure 1).1 With general substrates there are plenty of methods available to carry out this seemingly simple transformation- the paper provides a good summary of the state of the art in the introduction section. In the case of free amino acids, the amino group normally requires protection, obviously with non-acid sensitive groups. The problem with unprotected substrates is that their zwitterionic behaviour results in low solubility in organic solvents. The go-to conditions seem to be taking a slurry of the amino acid in tert-butyl acetate and adding stochiometric perchloric acid- a procedure originally described by Taschner et al in 1961.2 The latter is not a material you want anywhere near anything you’re doing in the lab. The obvious switcheroo to less hazardous acids (H2SO4, HNO3 etc.) doesn’t work (see Figure 2). The perchloric acid process is generally slow and frequently stalls irreversibly. Adding additional tert-butyl acetate or acid or both doesn’t re-invigorate the reaction.
The procedure described by Namba uses catalytic bis(trifluoromethanesulfonyl)imide in tert-butyl acetate and works well with free amino acids as well as carboxylic acids and alcohols (Figure 1).
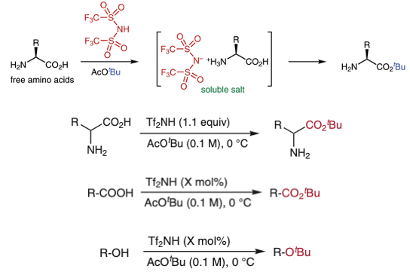
As a model substrate, reaction of 2-hydroxy-4-aminobutyric acid with 1.1 eqv of sulfonamide in tert-butylacetate gave a rapid reaction (2.5 hrs at 0°C, 86% yield). The equivalent perchloric acid process is much slower and lower yielding (16hrs, reaction stalled at 61% yield, Figure 2). Chiral integrity was maintained in both processes. As I highlighted above, just about all of the other acids tested (with the exception of trifluoromethane sulfonic acid, TfOH) didn’t work. TfOH, however, is not easily handled. It’s a fuming, water reactive liquid whereas the trifluoromethylsulfonamide is a solid (m.p. 52-56°C) making it that bit easier to handle.3 In addition the sulfonamide could be used sub-stoichiometrically with carboxylic acid and alcohol substrates.
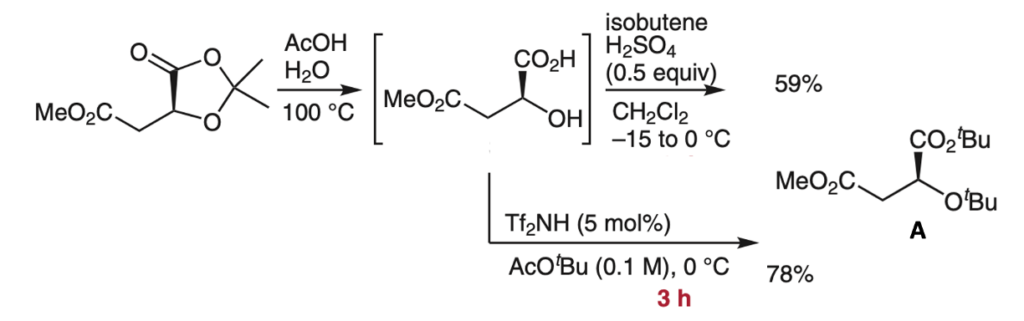
A final showcasing example from the paper is the conversion of an L-malic-acid derived acetonide to intermediate (A)- an fragment used in the synthesis of phytosiderophoric mugineic acids (Figure 3).4 The original conditions using isobutene gas and catalytic sulfuric acid required 7 days and gave a modest yield of 59% on gram scale. The analogous Tf2NH- catalysed process was complete in 3hrs at 0°C with a much-improved yield of 78%.
The second paper by Kim et al describes a one-pot process for conversion of your newly acquired tert-butyl ester directly to an amide or trans-ester.5 The benefit here is obviously avoiding having to cleave the ester using (most commonly) trifluoroacetic acid (TFA). As I described in an earlier post, this process is not easy to carry out at scale and is often replaced by a long neutralization process.6
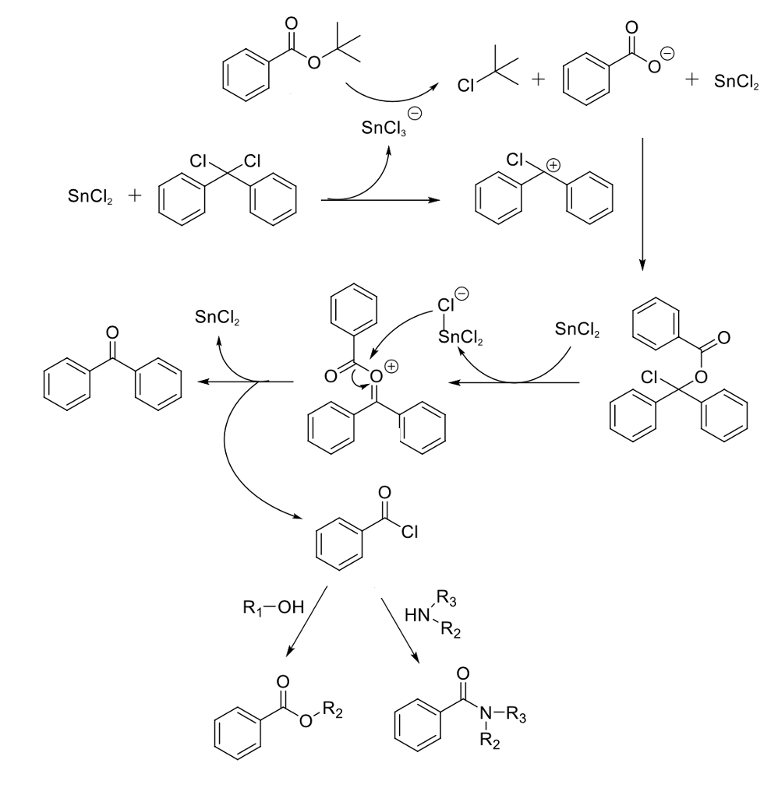
The gist of the reaction is in situ conversion of the tert-butyl ester to the acid chloride using dichlorodiphenylmethane with a Lewis acid (catalytic SnCl2), and trapping either with an amine (to give an amide) or an alcohol/thiol (to give another ester/thioester) (Figure 4).5 This combination of chloride source and Lewis acid was unique amongst the other reagents screened, with DIPEA being the best base for the second stage in the process- trapping the acid chloride. DMAP was added to the reaction involving alcohol substrates.
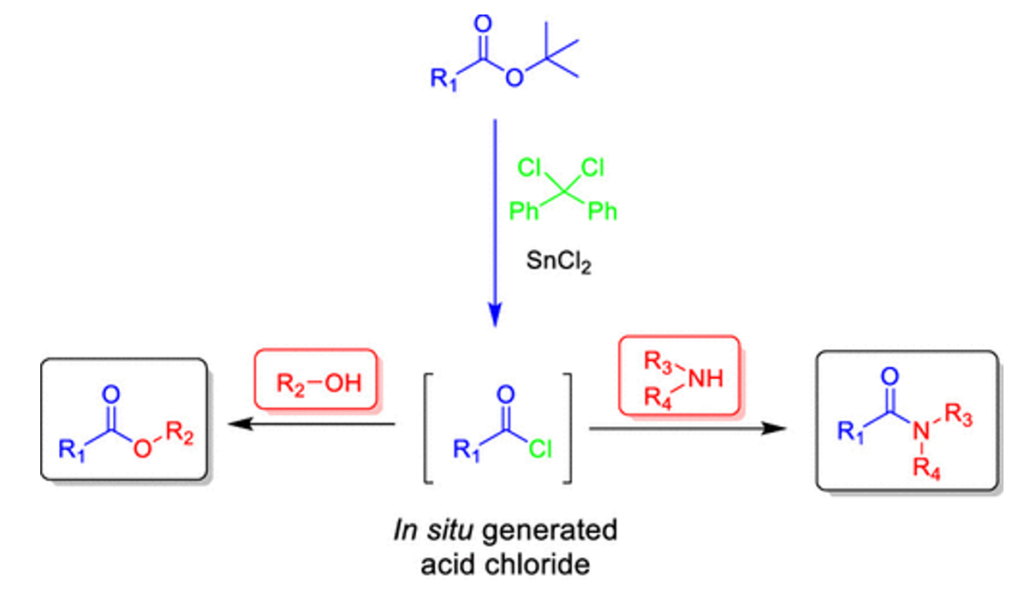
There are plenty of examples in the paper demonstrating the scope, though it must be said that the substrates are somewhat limited in their complexity. I guess this either means they don’t work or there’s another paper in the pipeline.
The mechanism is not discussed in great detail; however a plausible one is proposed (Figure 5). Dichlorodiphenylmethane and SnCl2 react to give SnCl3 – and a doubly benzylic carbocation. The tert-butyl group is cleaved generating tert-butyl chloride and a carboxylate anion that traps the carbocation. The rest of the sequence looks a little contrived but there’s nothing that suggests it’s not feasible.
A couple of papers that won’t change the world, but when it’s a problem your faced with could prove invaluable. It certainly would have been for me.
See you next time.
References:
- a) A simple and powerful tert-butylation of carboxylic acids and alcohols: K. Namba et al, Synlett 2024, 35, 235-239; b) The tert-butyl group in chemistry and biology: M. Muller et al, Org. Biomol. Chem. 2008, 6, 2665-2655
- Neue Veresterungsmethoden in der Peptidchemie, VIII. Darstellung von tert.-Butylestern freier Aminosäuren: E. Taschner et al, Liebigs Ann. Chem. 1961, 646, 134
- Hey Phenol- Everyone has their Cross to Bear: J. Studley blog article 2023, https://www.scientificupdate.com/process-chemistry-articles/hey-phenol-everyone-has-their-cross-to-bear/
- A practical synthesis of the phytosiderophore 2’-deoxymugineic acid: a key to the mechanistic study of iron acquisition by graminaceous plants: S. Kusumoto et al, Angew. Chem. Int. Ed. 2007, 46, 7060-7063
- Tin(II) chloride-catalyzed direct esterification and amidation of tert-butyl esters using a, a-dichlorodiphenylmethane under mild conditions: H. K. Kim et al, J. Org. Chem. 2023, 88, 13291-1330
- One Functional Group, So Many Functions: J. Studley blog article 2023, https://www.scientificupdate.com/process-chemistry-articles/one-functional-group-so-many-functions/








